RESEARCH ARTICLE
Clinical, Immunological and Virological Responses of Zidovudine-Lamivudine-Nevirapine versus Zidovudine-Lamivudine-Efavirenz Antiretroviral Treatment (ART) Among HIV-1 Infected Children: Asella Teaching and Referral Hospital, South-East Ethiopia
Abebe Sorsa*
Article Information
Identifiers and Pagination:
Year: 2018Volume: 12
First Page: 11
Last Page: 18
Publisher Id: TOMINFOJ-12-11
DOI: 10.2174/1874431101812010011
Article History:
Received Date: 11/10/2017Revision Received Date: 08/02/2018
Acceptance Date: 31/03/2018
Electronic publication date: 30/04/2018
Collection year: 2018
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
Antiretroviral Therapy(ART) remarkably reduced HIV-1 infection-related mortality in children. The efficacy and safety of different ART regimen in pediatric age groups remained issues of debates and available evidence were scarce especially among children taking the of one the two prototypes (NVP or EFV) Non-Nucleoside Reverse Transcriptase Inhibitor(NNRTI) as backbone of ART regimen.
Therefore, the objective of this study was to compare clinical, immunological and virological responses of zidovudine-lamivudine-nevirapine (AZT+3TC+ NVP) versus zidovudine-lamivudine-efavirenz (AZT+3TC+EFV) ART regimen among HIV-1 infected children.
Methods:
A retrospective cross-sectional study was done by reviewing medical records of the patients to evaluate clinical, immunological and virological outcomes of NVP+AZT+3TC versus EFV+AZT+3TC ART regimen among HIV-1 infected children. Data were entered into Epi-info version 7.2.2 for clean up and exported to SPSS version 17 for analysis. Paired and Independent t-tests were used to compare the CD4 cell count, weight and virologic level at six months with corresponding baseline value; and the mean weight, CD4 gain and viral suppression across the two ART regimens at six months of ART respectively.
Results:
Medical records of 122 patients from NVP-based regimen and 61 patients from EFV group were reviewed. After six months of NVP+AZT+3TC treatment, the mean CD4 cell count difference from baseline was 215(95% CI, 175.414-245.613, p<0.001). From EFV+AZT+3TC group, the mean CD4 cell count difference from baseline was 205(95% CI 155.404-235.623, p< 0.001). The mean CD4 count difference between the two regimens was comparable (p 0.145). Similarly, optimal viral suppression was achieved in 82% (100/122) of NVP+AZT+3TC regimen and 83% (44/61) of EFV+AZT+3TC regimen which was still comparable across the two groups.
Conclusion:
There was no difference in clinical, immunological and virological outcomes among patients taking NVP+AZT+3TC or EFV+AZT+3TC ART regimen.
1. INTRODUCTION
Antiretroviral Therapy (ART) for HIV-1 infection significantly suppresses viremia, improves CD4 count and reduces overall disease progression [1, 2]. The debate over which Non-Nucleoside Reverse Transcriptase Inhibitor (NNRTI) should be prescribed in combination with Antiretroviral Therapy (ART) for the treatment of HIV infection has been growing recently[3]. The standard therapy consists of two Nucleoside Reverse Transcriptase Inhibitors (NRTIs) and one non-nucleoside reverse transcriptase inhibitor (NNRTI). Nevirapine (NVP)–based ART has shown effective antiretroviral efficacy, even in patients with advanced HIV disease, and it has been widely used in resource-limited countries [4]. The controversy over which of the two NNRTI drugs should be started in combination with NRTI for the treatment of HIV infection has been growing recently [1, 3]. Previous studies comparing NVP-based and EFV-based regimens in adult patients have shown comparable effectiveness. However, some studies have shown the superiority of EFV. There are limited data comparing NVP to EFV in HIV-infected children. A study from the United States and Uganda found an increased risk of virologic failure for patients on NVP compared to EFV based regimen [5]. However, study from Nepal showed that patient taking NVP based regimen had superior clinical outcomes than EFV based regimen [6, 7]. Compared with a regimen of didanosine, lamivudine, and EFV, a regimen of once-daily didanosine, lamivudine, and NVP was inferior and was associated with more frequent virologic failure and death [8].
In this study, wedifferences at six months assessed clinical and immunological and virological outcomes of NVP+AZT+3TC versus EFV+AZT+3TC ART regimen among HIV-1 infected children.
2. METHODS
A cross-sectional descriptive study was conducted from May-August 2017 by reviewing ART documents of children infected with HIV-1 who were on ART at pediatric ART clinic, Asella Teaching and Referral Hospital (ATRH)
The hospital is one of the federal teaching and referral hospitals. Pediatric and child health department is one of the major departments delivering patient care service, teaching and research activities under different subunits categories. Pediatric ART clinic is one of the well-organized clinics of the hospital giving care for pediatric HIV/AIDS care and treatment.
2.1. Sample
At the time of data collection about 545 children with HIV infection were on follow up at pediatric infectious disease clinic. The sampling procedure was determined by patients’ ART regimen and to minimize sampling error and to control the effects of other NRTI patients taking AZT+3TC were included and matched under NVP+3TC+AZT versus EFV+3TC+AZT. Before and during the study period the utilization of EFV-based was low as the national guideline by then was not strongly recommending this regimen in pediatric age group. Accordingly for one patient included from EFV- based regimen two patients from NVP based regimen were included (1:2) making the sample size of (n1=61) from (EFV+3TC+AZT) and 122 from (NVP+3TC+AZT) (Fig. 1). A simple random sampling technique was used to select patient charts from each regimens using computer-generated random number.
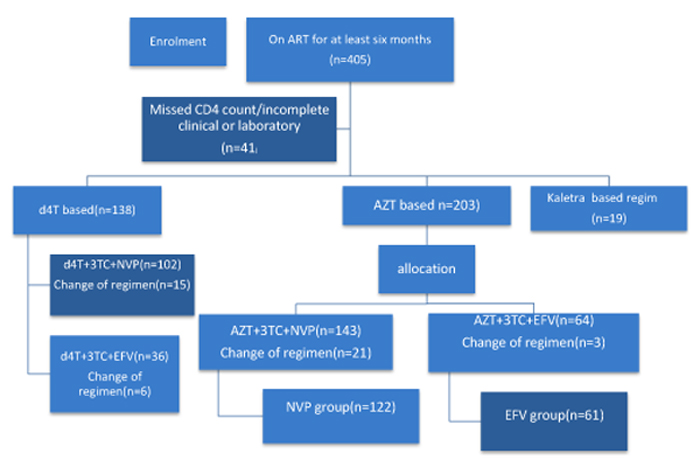 |
Fig. (1). Schematic flow chart demonstrating sampling technique, ATRH, 2017. |
2.2. Data Collection and Analysis
Data were collected from the patients' ART documents (medical records, ART log books, HMIS books) using standardized format. Data entry was made to EPI info software for clean up and analysis; exported to SPSS version 17 for further analysis. Paired t-test was used to compare the CD4 cell count, weight and virologic level at six months with corresponding baseline value and independent t-test was to compare the mean weight and CD4 gain at six months and viral suppression level at six between the two ART regimens.
The factors considered to affect the clinical and immunologic outcomes in both groups were assessed using binary logistic regression: baseline CD4%/count, WHO clinical staging, presence of chronic diarrhea, anemia, and baseline weight, occurrence of TB, and switching of ART regimen. 95% CI with p value of less than 0.05 was considered statistically significant.
2.3. Results
A total of 183 patients were included in the study. ART documents of 122 patients from NVP+3TC+AZT groups and 61 patients from EFV based regimen were reviewed. Males constitute 51% (62/120) while females were 49% (60/120) from NVP+3TC+AZT regimen. From EFV-based regimen, male comprised of 52% (32/61) and female 48% (29/61).
The youngest age at the initiation of ART was 5 months from NVP+3TC+AZT regimen and 17 months from EFV-based groups with the highest being 144 and 168 months (IQR age 74-104 and 95-130 months) respectively.
Baseline anthropometry showed that 20.4%(25/122) and 1.5% (2/122) from NVP+3TC+AZT regimen and 22% (14/61) and 2.5% (2/61) from EFV+3TC+AZT regimen were having moderate and severe wasting respectively.
Anemia was observed in 18.3% (22/122) of patients from NVP+3TC+AZT arm regimen and 14.8% (9/61) of EFV+3TC+AZT at the initiation of ART with the majority of the cases were having mild anemia.
About 69% (84/122) and 73% (44/61) patients were WHO clinical stage III or IV at the start of NVP+3TC+AZT or EFV+3TC+AZT regimen, respectively (Table 1).
| Variables | – | NVP+3TC+AZT n(%) |
EFV+AZT+3TC n(%) |
P value |
|---|---|---|---|---|
| Gender | Male | 62(59.1) | 32(52.0) | 0.605 |
| Female | 50(49.9) | 29(48.0) | ||
| Age category | Under 5 years | 41(35.7) | 10(15.7) | 0.021 |
| 5-14 years | 81(64.3) | 51(84.3) | ||
| Weight-for-age | <3rd centile | 63(51.7) | 32(52.3) | 0.056 |
| 3rd -97th centile | 59(48.3) | 29(47.7) | ||
| Weight –for-height | <3rd centile | 59(48.3) | 26(42.6) | 0.085 |
| 3rd -97th centile | 63(51.7) | 35(57.4) | ||
| WHO Clinical Staging of AIDS | I & II | 59(48.3) | 24(40.3) | 0.123 |
| III &IV | 63(51.7) | 37(60.7) | ||
| CD4 cell category | >25% or >500 | 9(7.5) | 5(8.2) | 0.115 |
| 15-25% or 200 – 500 | 46(37.5) | 31(50.8) | ||
| <15% or <200 | 67(55) | 25(41) | ||
| Base anemia | – | 22(18.3) | 9(14.8) | 0.095 |
The CD4 cell count at the start of HAART ranged from 15-2000 cell/ml with an Interquartile Range (IQR) of 235-325 cell/ml among NVP+3TC+AZT. And among patients taking EFV+3TC+AZT based ART regimen, baseline CD4 count ranged 25-2075 cell/ml with an IQR range of 260-355 cell/ml.
The CD4 cell count at six months of ART was compared with baseline and among the two groups. From NVP+3TC+AZT based ART regimen the CD4 cell count range from 75-2400 c/ml with IQR of 468-632 c/ml, and mean CD4 cell count difference of 215, 95% CI (175.414-245.613). From EFV based group, CD4 count ranged from 65-2100 c/ml with IQR of 435-605 c/ml, and the mean CD4 cell count difference of 205, 95% CI (155.404-235.623). The mean CD4 count differences at six months of ART were comparable between the two groups (mean CD4 17.32, 95%CI 15.55-57.55, p 0.123)
Patients who have been on NVP+3TC+AZT regimen gained mean weight of 0.78kg and 1.81kg with 95% CI (0.588-0.957 and 1.60-2.2 respectively) at three and six months of HAART. Similarly, the mean weight gain at three and six months of EFV+3TC+AZT were 0.75 and 1.70kg with CI (0.588-0.954) and 1.70kg, 95% CI (1.50- 1.95). Still, there is no significant difference in weight gain among the two groups (Table 2).
| – | – | NVP+3TC+AZT n(%) | EFV+AZT+3TC n(%) | Chi2 p value |
|---|---|---|---|---|
| – | Category | – | – | – |
| Weight gain(%) at three months | <5% | 71(58.3) | 37(60) | 0.524 |
| 6 -10% | 24(20) | 13(21.7) | 0.752 | |
| >10% | 21(21.7) | 11(18.3) | 0.245 | |
| Weight gain(%) at six months | < 5% | 42(34) | 20(33.3) | 0.105 |
| 5-10% | 27(22) | 14(23.3) | 0.169 | |
| >10% | 53(44) | 27(43.4) | 0.306 | |
| CD4 cell level | >25% or >500 | 49(40) | 24(40) | 0.315 |
| 15-25% or 200 – 500 | 63(51.6) | 31(50) | 0.544 | |
| <15% or <200 | 10(8) | 6(10) | 0.172 | |
| Viral load | <1000 copy/ml | 100(82%) | 51(83.3) | 0.341 |
| Tuberculosis(TB) | 13(10.8) | 6(10) | 0.413 | |
| Switching of ART regimen | 23(12.8) | 4(6.7) | <0.001 |
After six months of ART 82% (100/122) from NVP+3TC+AZT and 83%(44/61) from EFV+3TC+AZT were having adequate viral suppression(<1000coppy/ml) (Fig. 2).
 |
Fig. (2). Immunological and virological outcomes after six months of ART at ATRH, 2017. |
During the first six months of HAART, a total 27 patients developed tuberculosis with comparable incidence across the two groups (Table 2).
With regards to change of HAART during the first six months of treatment; 23(12.8%) patients who were on NVP+3TC+AZT HAART regimen were switched to other line of ART regimen while 4(6.7%) patients from EFV-based regimen switched to other regimen indicating ART switching was more commonly seen among NVP+3TC+AZT(p<0.001).
Early clinical stage of the disease and mild immune suppression at the start of ART were predicting favorable clinical, immunological and virological outcomes ART among the two groups of patients (see Table 3). Similarly, normal baseline weight, absence of diarrhea and weight gain >10% at three and six months of ART were found to be a good prognostic indicator of clinical, immunological and virological outcome regardless of the ART regimen (see Table 3).
| Variables | CD4 cell increment by >50% | Viral load <1000copy /ml | |||
|---|---|---|---|---|---|
| N=123, AOR with (95% CI) | P-value | N=152 AOR with (95% CI) |
P-value | ||
| Weight category | 3rd-97th centile | 3.22(1.09-6.56) | 0.018 | 3.29(1.91-5.71) | <0.001 |
| Sex | Male | 1.02(0.9-1.5) | 0.073 | 1.06(1.14-2.15) | 0.07 |
| WHO clinical Staging | I | 4.76(1.53-12.15) | 0.014 | 1.67(1.24-2.24) | <0.001 |
| II | 2.08(1.10-5.65) | 0.006 | 1.21(0.92-1.61) | 0.018 | |
| Base line CD4 countc | 15-25%/200-500 cell/ml | 4.35(1.47-11.24) | 0.025 | 1.73(1.25-2.40) | <0.001 |
| >25%/>500 cell/ml | 1.79(0.56-6.25) | 0.015 | 1.81(1.38-2.38) | <0.001 | |
| Aged | <5 years | 2.13(0.81-9.09) | 0.017 | 2.06(1.46-2.91) | <0.001 |
| Base line (Hgb)e | >10g/dl | 2.94(1.02-7.14) | 0.014 | 5.88(2.60-13.28 | <0.001 |
| Weight gain(%) at three month of ARTf | >10% | 2.5(1.3-4.9) | 0.012 | 11.32(7.37-17.40) | <0.001 |
| Weight gain(%) at six month of ARTf | >10% | 4.23(2.12-7.2) | <0.001 | 3.39(1.92-5.72) | <0.001 |
| Chronic diarrhea | No | 2.56(0.69-7.25) | 0.007 | 1.76(1.14-2.15) | 0.005 |
| ART regimen | NVP+AZT+3TC | 1.05(1.045-2.5) | 0.086 | 1.07(1.24-2.24) | 0.12 |
3. DISCUSSION
In this study, after six months of NVP+3TC+AZT ART regimen the mean CD4 cell count increment was 215/ml, and 205/ml from EFV+AZT+3TC group the mean CD4 cell with no significant difference among the two groups which is in agreement with report from adult HIV- infection clinical trial which showed no significant differences between the groups with respect to the change in the CD4 cell count from base line [1]. Similarly, ART regimen variable was not significantly associated with recovery of CD4 counts [2, 9, 10, 11]. However; another study from Uganda reported higher mean CD4 increment among patients taking EFV based ART than their NVP-based counterparts even if NRTI back bone was not matched in this study and the study was conducted among adult patients [5].
This study also demonstrated optimal virological suppression (<1000copy/ml) in 82%(100/122) and 83%(44/61) among NVP+3TC+AZT and EFV+AZT+3TC respectively. This is incongruent with WHO recommendation which highlighted no evidence of EFV virologically superior to NVP [12]. Likewise; a cochrane review of seven randomized clinical trials demonstrated that the two drugs provided comparable levels of viral suppression in non-TB patients infected with HIV when combined with two nucleoside reverse transcriptase inhibitors [7]. How ever; in a meta-analysis patients in the EFV+AZT+3TC treatment group achieved statistically higher rates of virological response and low risk of viral failure, likely due to the effects of rifampin on NVP metabolism while a study from Nepal showed EFV based regimen had less virus suppression effects than NVP-based [6, 13, 14]. Another cohort study in Thai adult patients with advanced HIV infection showed that NVP+3TC+AZT and EFV+AZT+3TC ART regimens were equally effective in terms of virological and immunological responses [5, 15, 16, 17, 18].
Presence of chronic diarrhea during ART treatment is associated with poor CD4 cell recovery independent of the nutritional status of the patient which is in agreement with most previous studies. The reason was not clearly identified so far but could be because of associated mal-absorption and poor adherence during diarrheal episode. This research also uncovered higher baseline CD4 cell value strongly predicted good six month CD4 cell recovery and viral suppression response in both groups of patients which was supported with a large cohort study involving 861 adult patients living with HIV in Spain that showed patients with baseline CD4 count of 200 and of 201 to 350 cells/mm3 had a significantly lower chance of achieving CD4 count of 500 cells/mm3 compared with patients with baseline CD4 350 cells/mm3 and above [19].
This study also showed optimal weight recovery after initiation of ART strongly associated with better CD4 response and viral suppression which is in line with study report from South Africa which elaborated lower percentiles of weight gain after six months of ART were associated with poor subsequent treatment outcomes and a higher risk of mortality independent of other baseline characteristics. Likewise, normal weight at the start of ART was independent predictors of favorable immunological and virological outcomes in both wings which was coinciding with other observational studies where patients who were underweight had a two-fold increased risk of poor treatment outcomes [20, 21]
CONCLUSION
This research finding concluded that there is no difference in clinical, immunological and virological outcomes among patient taking NVP+AZT+3TC versus EFV+AZT+3TC regimen. The conflicting results from different studies could be explained by study design, patient selection and possible concomitant HIV-TB treatment which could significantly affect the outcomes.
LIST OF ABBREVIATIONS
| AIDS | = Acquired Immunodeficiency Syndrome |
| ART | = Antiretroviral Treatment |
| ARV | = Antiretroviral |
| ATRH | = Asella Teaching and Referral Hospital |
| AZT | = Zidovudine |
| EFV | = Efavirenz |
| HAART | = Highly Active Antiretroviral Therapy |
| HIV | = Human Immune-Virus |
| MTCT | = Mother To Child Transmission |
| NNRTI | = Non Nucleoside Reverse Transcriptase Inhibitors |
| NRTI | = Nucleoside Reverse Transcriptase Inhibitors |
| NVP | = Nevirapine |
| PMTCT | = Prevention Of Mother To Child Transmission |
| SD | = Standard Deviation |
| TB | = Tuberculosis |
| WHO | = World Health Organization |
| 3TC | = Lamivudine |
FUNDING SOURCE
As I sponsored the research project myself for all parts of research work, there is no conflict of interest from anybody.
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
Ethical clearance was obtained from the department of Institutional Review Board of Arsi University College of Health Science. Letter of permission was given from the department of pediatrics and child health to collect medical records. The records of patients were kept at a safe place and information collected from the record was kept confidential.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are base of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The author declares that there is no competing and conflict of interest from any individual and organization.
ACKNOWLEDGEMENTS
I would like to express my heartfelt gratitude to Arsi University College of Health Science, department of pediatrics and child health for allowing me to conduct the study. I would like to extend my heartfelt acknowledgement to the record office of pediatric ART clinic for their immense contribution during data collection.




